|
About 60% of the Earth's crust, 20% of the air we breathe, and 26%
of all living matter are made up of oxygen atoms. How the different
elements react when brought close to oxygen is an important part
of their chemical behavior. Water, the medium in which life evolved,
can be thought of as a medium for bringing oxygen atoms close to
other substances.
All of the elements that we have discussed so far, with the exception
of helium and neon, form compounds with oxygen, called oxides. Oxides
of metals are bases, and oxides of nonmetals are acids. The reason
for this difference in behavior lies in the electronegativities
of the atoms bound to oxygen.
|
•
• |
Oxides of metals are bases
Oxides of nonmetals are acids
|
|
|
The second-shell elements run the gamut of electronegativity from
1.0 (Li) to 4.0 (F). When oxides of these elements are dissolved
in water, bonds are formed of the general type
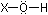
in which X is a second-shell element. (Outer-shell electron pairs
on oxygen atoms will be shown explicitly in the following discussion.
Remember that a chemical bond line as in X-O also represents an
electron pair.)
|