|
LiF is soluble in water because the polar water molecules attack
and hydrate the ions as shown above. It is not soluble in nonpolar
liquids because nonpolar molecules show no attraction for the ions
and are of no help in pulling the crystal structure apart.
Many salts are soluble in water but almost none are soluble in nonpolar
liquids such as benzene, gasoline, or carbon tetrachloride, 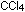 . .
|
|
You can drop a small amount of table salt, NaCl, into a glass of
water and see the crystals dissolve and disappear quickly. But crush
the salt crystals as fine as you like, and after being mixed with
olive oil (nonpolar) they will remain as a white, insoluble powder.
|