|
LiF behaves entirely differently. The difference in electronegativity
between the two atoms is so great that a diatomic LiF molecule is
in a highly unfavorable state. There is a strong tendency for the
electron pair to be given over completely to the F atom, thereby
turning the molecule into two ions:

(The dots represent outer-shell electrons, and the bond line between
atoms represents an electron pair.)
When this occurs, each  ion attracts all the
ion attracts all the 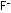 ions present, and each
ions present, and each 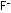 attracts every
attracts every  .
The gas of separate Li-F molecules condenses into a liquid of .
The gas of separate Li-F molecules condenses into a liquid of  and
and 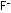 ions (see next paget) when the temperature decreases below 1676°C.
ions (see next paget) when the temperature decreases below 1676°C.
Only above this temperature does LiF have enough energy to force
the electron pair back toward the  ion and turn the separate ions into gaseous Li-F molecules.
ion and turn the separate ions into gaseous Li-F molecules.
|
|
|