|
In Chapter 4 we saw examples of equal sharing of electrons in a
bond (methane), unequal sharing (ammonia, water, HF), and partial
or complete transfer of electrons to form ions (HF and LiF in water).
Ions and salts were introduced primarily as a contrast to electron-sharing.
Now we turn to the behavior of ions as they are encountered in salts,
solutions, and metals.
Acids and bases were introduced in Chapter 4 with examples of each:
the acid HF and the bases 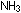 and LiOH. Some of the most common and useful acids and bases are
oxygen compounds.
and LiOH. Some of the most common and useful acids and bases are
oxygen compounds.
In this chapter we shall look at oxygen compounds of the second-shell
elements and see why electronegativity differences make some of
them acids and others bases. Why, for example, does lithium hydroxide
(LiOH) behave so differently from nitric acid 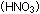 ? ?
Finally, although the brittle salts and flexible metals both are
made from ions, they are as different in properties as two solids
can be.
|
|
|