|
Theory
The reaction that occurs is:
(NH ) ) Cr Cr O O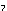 (s) (s)
 Cr Cr O O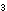 (s)
+ N (s)
+ N (g) + 4H (g) + 4H O(l) O(l)
Further details
The value for the standard molar entropy of ammonium dichromate
has been estimated by Latimer's rules, which state that the entropy
of each atom of each element in a compound in JK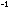 mol
mol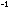 is given by:
is given by:
 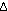 S S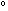 = (1.5 R ln
= (1.5 R ln 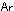 )
- 3.92 )
- 3.92
(where 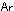 is the
relative atomic mass of the element and R is the gas constant) and
that the entropy of a compound is the sum of the entopies of all
elements in the compound. is the
relative atomic mass of the element and R is the gas constant) and
that the entropy of a compound is the sum of the entopies of all
elements in the compound.
This rule may be found useful for estimating the entropies of other
compounds which are not readily available in the literature.
|
|
Safety
Wear eye protection.
Dispose of the residue in a sealed plastic bag placed in the dustbin.
|