|
4. (a) Inflate a balloon with carbon dioxide gas from a cylinder.
Attach the balloon securely over the mouth of a test-tube and immerse
the tube in liquid nitrogen. The balloon will deflate as the gas
solidifies and the tube will be filled partially with solid carbon
dioxide.
(b) Inflate a balloon with carbon dioxide gas from a cylinder. Place
the balloon in a container of liquid nitrogen, or pour liquid nitrogen
over it. The balloon will collapse as the CO solidifies. Cut the balloon open with scissors to reveal a frost
of dry ice inside.
solidifies. Cut the balloon open with scissors to reveal a frost
of dry ice inside.
5. Pour 1cm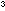 of water into a test-tube and mark the level that it reaches. Empty
and dry the tube and cool it by immersion in liquid nitrogen. Pour
about 2cm
of water into a test-tube and mark the level that it reaches. Empty
and dry the tube and cool it by immersion in liquid nitrogen. Pour
about 2cm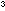 of liquid nitrogen into the tube and hold the tube in liquid nitrogen
to prevent the liquid in the tube from boiling away until ready
for the next step.
of liquid nitrogen into the tube and hold the tube in liquid nitrogen
to prevent the liquid in the tube from boiling away until ready
for the next step.
Take the test-tube out of the liquid nitrogen and allow the liquid
inside to boil away until the level reaches the 1cm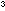 mark. Now secure a balloon over the mouth of the tube and let the
remaining 1cm
mark. Now secure a balloon over the mouth of the tube and let the
remaining 1cm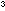 of liquid nitrogen boil away, inflating the balloon. When all the
liquid is boiled away, remove the balloon and tie it, taking care
not to lose any gas.
of liquid nitrogen boil away, inflating the balloon. When all the
liquid is boiled away, remove the balloon and tie it, taking care
not to lose any gas.
|
|
Estimate the volume of the inflated balloon from its diameter or
by immersing it in a bowl of water and measuring the increase in
water level. One cm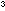 of liquid nitrogen gives about 2dm
of liquid nitrogen gives about 2dm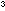 of gas.
of gas.
Any remaining liquid nitrogen can be disposed of as follows:
(a) Pour some into a plastic fish tank of water containing a little
washing up liquid and a few drops of food dye. A spectacular fog
will be produced and frozen bubbles will be left behind.
(b) Pour a little onto the floor close to the feet of the audience
to allow them to experience the coldness as it boils away.
Liquid nitrogen is constantly boiling at room temperature and pressure
as it is almost 200K above its melting point. Point out the extra
vigour with which it boils when an object at room temperature is
placed in it. This is comparable to putting a hot poker into water.
Safety
Wear eye protection and use insulating gloves when handling
liquid nitrogen. Care is needed when handling liquid oxygen and
some teachers may prefer to omit this part of teh experiment.
|