|
Visual Tips
A white background will help. For a large audience the effect
is clearer if the reactions are scaled up and done in beakers.
Theory
The equilibrium is:
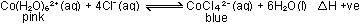
The shifts in equilibrium position produced both by temperature
and concentration changes are in accordance with Le Chatelier's
principle.
|
|
Extensions
It is possible to show that it is the 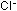 ions in the hydrochloric acid that shift the equilibrium by adding
a spatula of sodium chloride to the pink solution. This produces
a bluer colour eventually although this takes some time to form
because the salt is slow to dissolve.
ions in the hydrochloric acid that shift the equilibrium by adding
a spatula of sodium chloride to the pink solution. This produces
a bluer colour eventually although this takes some time to form
because the salt is slow to dissolve.
Safety
Wear eye protection.
|