|
The Demonstration
Weigh about 50g of sucrose (ordinary table sugar) into a 100cm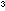 beaker (this is about half a beakerful). Stand the beaker on a large
watch glass in a fume cupboard. Pour onto the sugar about 20cm
beaker (this is about half a beakerful). Stand the beaker on a large
watch glass in a fume cupboard. Pour onto the sugar about 20cm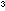 of concentrated sulphuric acid.
of concentrated sulphuric acid.
The sugar will turn yellow, then brown and after about a minute
it will start to blacken and a spongy mass of carbon will begin
to rise up the beaker and steam will be evolved. The carbon will
eventually rise to two or three times the height of the beaker.
The steam can be tested with cobalt chloride paper which will turn
from blue to pink. Sulphur dioxide is also given off and this will
turn potassium dichromate paper from orange to blue-green.
The beaker becomes very hot. If one drop of water is squirted from
a wash bottle onto the outside of the beaker, the drop will steam.
|
|
Place about 3g of blue hydrated copper sulphate on a watch glass
and pour onto it about 2cm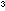 of concentrated sulphuric acid. Over a period of about three minutes
the colour will change to white as the acid dehydrates the salt.
Heat is evolved. The change can be reversed by adding water.
of concentrated sulphuric acid. Over a period of about three minutes
the colour will change to white as the acid dehydrates the salt.
Heat is evolved. The change can be reversed by adding water.
Paper and wood shavings can also be dehydrated with sulphuric acid.
|