|

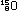 
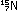 
     
    
 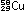
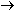 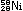
 
    
    
Alternatively, the same result can be obtained in other nuclei by
capturing an electron orbiting around the nucleus and using it to
convert a proton to a neutron. This is electron capture:
 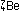
 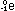
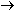 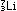
    
    
|
|

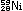
 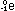
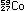
    
    
Many nuclei can decay by either positron emission or electron
capture. Both correspond to a movement down and to the right
by one step in the stability graph, thereby bringing the nucleus
closer to the stable region. In all of the reactions we have
seen so far - beta emission, positron emission, and electron
capture - notice that both mass number and charge are conserved.
|