|
In
this region, beyond atomic number 26, energy is released by fission
rather than fusion. At the far left of the curve, hydrogen fusion
in stars releases energy:
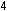 
 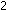 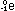
 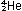
 
    
    
    
    
and at the far right, uranium fission in atomic reactors
also releases energy:
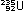 
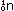 
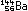 
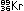 
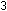 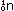
 
    
    
(This is only one of many ways in which the 235U nucleus can break down.)
The maximum stability of the iron nucleus is the reason why the
element-building process by successive fusion reactions, outlined
in Chapter 8, stops at iron. Beyond iron the fusion process is energy-requiring
instead of energy-yielding. The heavier elements are built up by
more indirect processes involving neutron capture.
In spite of the fact that mass is not conserved in nuclear reactions,
conservation principles do apply to the total number of heavy
particles (protons and neutrons) and total charge. This is implicit
in the equations for the two previous nuclear reactions.
|

|

