|
Example.
What is the mass loss per nucleon for the 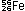 atom, compared with its component protons, neutrons, and electrons?
atom, compared with its component protons, neutrons, and electrons?
Solution.
The 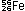 atom contains 26 protons, 26 electrons, and 30 neutrons, so the
mass calculation is performed as opposite:
atom contains 26 protons, 26 electrons, and 30 neutrons, so the
mass calculation is performed as opposite:
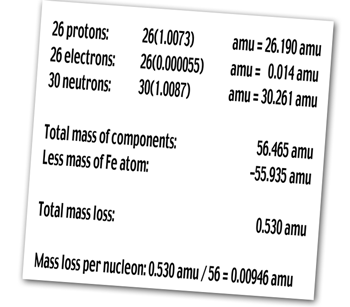
Notice that the mass loss per nucleon, and hence the binding energy
per nucleon, is greater for iron than for helium. This means that
the iron nucleus is more stable relative to protons and neutrons
than the helium nucleus is. If some combination of helium nuclei
could be induced to produce an iron nucleus, energy would be given
off, which would correspond to the increased stability of the product
nucleus per nuclear particle.
|
 |
