|
Exercise.
For practice, balance the following reactions by both the oxidation-number
and half-reaction methods:
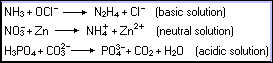
Although half-reactions have been introduced here merely as a means
of obtaining a balanced overall reaction, they can have physical
meaning of their own. If the iron oxidation and manganese reduction
can be carried out in separate containers, and if these containers
can be given suitable electrical connections, then we can make use
of the energy released by this reaction as the electrons flow through
a wire from the iron container to the manganese. This is the principle
of the electrolytic cell or battery. An ordinary flashlight battery
uses manganese reduction, and oxidation of the zinc battery casing.
We will return to electrolytic cells and energy production in Chapter
17.
|

|
