|
In
chemical processes, the most important property to be conserved
is the number of atoms of each kind that are present. Unlike nuclear
processes, chemical reactions do not create or destroy atoms, or
change one kind of atom into another. They only reshuffle the atoms
that were originally present into different molecular combinations.
What we would like to be able to do is to count each kind of atom
before and after a reaction and make sure that none has been gained
or lost.
Counting atoms directly is not practical, but because mass-energy
conversion is negligible in chemical reactions, conservation of
the number of atoms effectively means the conservation of mass.
From the discussion of moles and Avogadro's number in Chapter 2,
we know that the mass of a substance divided by its atomic or molecular
weight is the quantity of the substance in moles, and that one mole
of any chemical substance contains the same number of particles.
That number is Avogadro's number, N, which is 6.022 x 10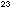 particles per mole. Hence by weighing moles of a substance, we effectively
can count atoms with a laboratory balance.
particles per mole. Hence by weighing moles of a substance, we effectively
can count atoms with a laboratory balance.
The symbols in a properly balanced chemical equation tell much more
than just the identity of reactant and product molecules. The equation
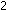 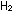    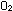    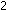 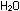
    
    
    
|

|