|
1. Natural lithium is 92.58% lithium-7, with atomic weight 7.016,
and 7.42% lithium-6, with atomic weight 6.015. What is the observed
atomic weight of lithium as it occurs in nature?
2. The observed atomic weight of chlorine in nature is 35.453,
and 75.53% of this is chlorine-35, with an atomic weight of 34.97.
If there is only one other chlorine isotope, what is its approximate
atomic weight?
3. The formula for methyl alcohol is CH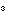 OH.
What is its molecular weight? OH.
What is its molecular weight?
4. At 120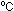 ,
which weighs more, a mole of water vapor or a mole of rnethyl alcohol
(also a gas at this temperature)? Which occupies more volume? Calculate
the weight and volume of each. How many molecules are in each? ,
which weighs more, a mole of water vapor or a mole of rnethyl alcohol
(also a gas at this temperature)? Which occupies more volume? Calculate
the weight and volume of each. How many molecules are in each?
5. How many oxygen atoms are present in one mole of 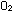 molecules? How many moles of oxygen atoms are present in a mole
of
molecules? How many moles of oxygen atoms are present in a mole
of 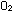 molecules?
molecules?
6. How many grams of oxygen are involved with 50 grams of
carbon in methyl alcohol? How many moles of oxygen atoms does this
correspond to?
|
|