|
The ideal gas law describes the behavior of a fictional gas.
Real gases act at room temperature as if they would shrink to nothing
at absolute zero, when in fact they condense first. Before reaching
absolute zero, all real gases liquefy or solidify, behavior for
which the ideal gas law cannot account.
No gas obeys the conditions PV = nRT perfectly, but all gases
come close at room temperatures and low pressures. This is the reason
that we can apply the gas law to any gas, including an atmospheric
mixture of 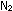 and
and 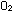 , without
worrying about the composition of the mixture. One molecule is the
same as any other in an ideal gas. , without
worrying about the composition of the mixture. One molecule is the
same as any other in an ideal gas.
The ideal gas law assumes that attractions between molecules are
negligible when compared with their energies of motion, and that
the actual volumes of gas molecules are negligible in comparison
with the total volume occupied by the gas.
|
|
This is close to being
true at room temperature and 1 atm pressure.
At lower temperatures and slower speeds, the attractive forces between
molecules no longer can be ignored.
At higher pressures, at which molecules are closer together, the
volume occupied by the molecules themselves becomes an appreciable
part of the volume filled by the gas. The ideal gas law begins to
fail badly.
Nevertheless, under ordinary conditions the expression PV=nRT
is a surprisingly good description of real gas behavior.
|