|
Charles' data suggest
that, if a gas continued to behave at lower temperatures in the
way that it does at room temperature, its volume would shrink to
nothing at -273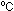 .
This is the point at which, in principle, all molecules would come
to rest and gases would cease to exert pressure or occupy volume. .
This is the point at which, in principle, all molecules would come
to rest and gases would cease to exert pressure or occupy volume.
This theoretically possible but experimentally unattainable temperature
is known as absolute zero. We can define an absolute temperature
scale (also known as the Kelvin scale after the British thermodynamicist
Lord Kelvin), in which the temperature (T) in degrees absolute or
Kelvin (K) is related to the temperature in degrees centigrade (t)
by the expression
 T(K)
= t ( T(K)
= t (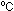 )
+ 273.15 )
+ 273.15 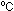
Top Right: Jacques Charles (1746 - 1823)
Bottom Right: Lord Kelvin (1824 - 1907)
|


|

