|
Solution 2. Use Boyle's law in the form P V V =
P =
P V V ,
where conditions (1) are at sea level and (2) are at the higher
altitude: ,
where conditions (1) are at sea level and (2) are at the higher
altitude:
 (1atm)
(7600 liters) = (0.70 atm) V (1atm)
(7600 liters) = (0.70 atm) V

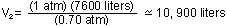
A drop in pressure to 0.70 atm has permitted the gas in the balloon
to expand.
Right: Sir Robert Boyle (1627 - 1691)
|
