|
Most chemical measurements are made in grams. An amount of any substance
in grams that is numerically equal to its atomic or molecular weight
in amu has been defined as one mole of that substance.
By this definition, one mole of hydrogen is 2.016 grams, one mole
of methane is 16.043 grams, and one mole of water is 18.015 grams.
We can convert any gram quantity of a chemical substance to moles
by dividing by its molecular weight. Once we have done this, we
know that equal numbers of moles of all kinds of substances must
have equal numbers of molecules. The same number of molecules is
present in a mole of hydrogen, water, methane, or any other substance.
This is very useful, because then we can measure the right amounts
of starting material for chemical reactions, and can tell from the
results how many molecules of product were formed per molecule of
reactants.
Example. How many moles of carbon are present in the 100
g of the preceding example? How many moles of hydrogen atoms would
be needed to combine with these? How many grams of hydrogen would
be needed?
|
|
Solution. The number of moles of carbon is
(100g carbon / 12.011 g 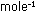 )
= 8.33 moles of carbon )
= 8.33 moles of carbon
Four times as many hydrogen atoms are needed as carbon atoms to
make methane, 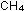 ,
so four times as many moles will be required also: ,
so four times as many moles will be required also:
(4 moles H / 1 mole C) x 8.33 moles C = 33.3 moles of hydrogen
Since the atomic weight of hydrogen is 1.008, this corresponds to
33.3 moles hydrogen x 1.008 g 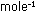 = 33.6g of hydrogen
= 33.6g of hydrogen
This is the same answer as we obtained previously, but this time
we used moles instead of merely the ratio of atomic weights.
|