|
The
reaction on the previous page was written using the 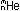 notation introduced previously, with the subscript now representing
the charge on a particle (rather than the number of protons in the
nucleus), and the superscript giving the mass number, or approximate
mass in amu.
notation introduced previously, with the subscript now representing
the charge on a particle (rather than the number of protons in the
nucleus), and the superscript giving the mass number, or approximate
mass in amu.
|
|
A
proton, which has a +1 charge and unit mass number, is written 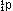 ;
a neutron, which has no charge and unit mass, is ;
a neutron, which has no charge and unit mass, is 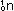 ;
and an electron, which has a - 1 charge and no negligible mass on
this rough-counting scale, is ;
and an electron, which has a - 1 charge and no negligible mass on
this rough-counting scale, is 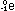 .
When a nuclear reaction such as this one is written and balanced
properly, the total charge (subscripts) and the total mass number
(superscripts) on the left must equal the total charge and mass
on the right. .
When a nuclear reaction such as this one is written and balanced
properly, the total charge (subscripts) and the total mass number
(superscripts) on the left must equal the total charge and mass
on the right.
|