|
An atom is made up of a very small but heavy central nucleus with
a positive charge, surrounded by a negatively charged cloud of electrons.
Because atoms are so small, the familiar units of feet or centimeters
are useless in measuring them. A more common unit of length is the
angstrom, symbolized 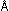 . .
There are 100,000,000 or 10 in one centimeter, or to express matters the other way around,
in one centimeter, or to express matters the other way around,
1 = 1/10 cm = 10
cm = 10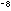 cm = 0.00000001 cm
cm = 0.00000001 cm
Most atoms are of the order of 1.0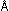 to 2.4
to 2.4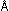 in diameter, which is why angstroms are so convenient. The nucleus
of an atom is much smaller yet, typically with a diameter of 10
in diameter, which is why angstroms are so convenient. The nucleus
of an atom is much smaller yet, typically with a diameter of 10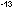 cm or 10
cm or 10 .
.
If an atom were as large as a football stadium, the nucleus would
be the size of a small ladybug crawling across the 50-yard line.
In spite of this size difference, virtually an of the mass of an
atom is concentrated in its nucleus. One electron, which has a negative
charge, weighs only 1/1836 as much as the lightest of all nuclei,
that of the hydrogen atom (proton).
|
|