|
1.
If covalent bond radii are additive, what should the distance between
centers of carbon and hydrogen atoms be in methane, 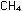 ?
This distance is called the carbon-hydrogen single bond length. ?
This distance is called the carbon-hydrogen single bond length.
2. What should the carbon-oxygen and oxygen-hydrogen bond lengths
be in methanol (also called methyl alcohol), which has the bonding
structure shown opposite?
3. Lithium atoms in the metal are packed together in such a way
that they take up 68% of the available volume, with the rest being
empty space between atoms. Therefore an atom of actual volume V
= 4 / 3  r r
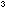 ,
in which r is the atomic radius, would account for V'
= V / 0.68 of crystal volume per atom. From the crystal volume
per atom calculated for lithium in the text, calculate the actual
radius of a lithium atom in the metal. How does this compare with
the rough figure arrived at in the text, and with the tabulated
metallic radius for lithium seen previously in the chapter? ,
in which r is the atomic radius, would account for V'
= V / 0.68 of crystal volume per atom. From the crystal volume
per atom calculated for lithium in the text, calculate the actual
radius of a lithium atom in the metal. How does this compare with
the rough figure arrived at in the text, and with the tabulated
metallic radius for lithium seen previously in the chapter?
|
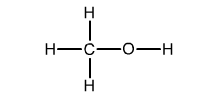
methanol
|
