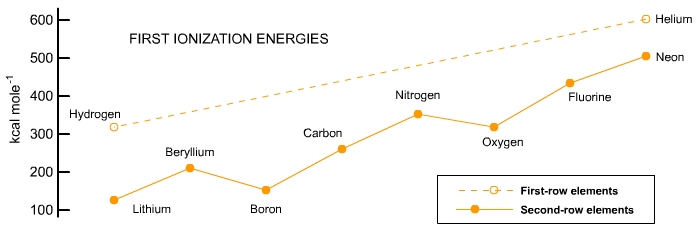
 |
||
|
First IE's for the second-shell atoms are lower because the electrons are farther from the nucleus and are shielded by the inner electron pair. They generally increase with atomic number because the nuclear charge increases. For elements at the far right of the graph, it is nearly as hard to take an electron away from a neon atom with a closed eight-electron shell as it is to remove one electron from helium with its closed two-electron shell. |
What does ionization energy have
to do with chemical behavior? The low IE of lithium is characteristic
of a metal, and causes it to form salts and other compounds
in which lithium exists as a positively charged Li |
|