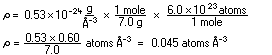|
Democritus, Newton, and Dalton
all used atoms as philosophical ideas without worrying about the
actual size of one such particle of matter. They would not have
known how to begin to calculate the size of an atom. We can find
out roughly how big a typical atom is with only a short scratch-paper
calculation. The density of lithium metal is measured to be 0.534
g cm |
|
|